Introduction: Cancer-associated cachexia is associated with poor survival rates in several cancer types. Multiple Myeloma (MM) preferentially occurs in the elderly and is the most common cancer that causes lytic bone metastasis causing bone pain, fractures, mobility limitations, and falls. Cachexia is projected to be common in myeloma but has not been fully studied. Myeloma treatments including corticosteroids, radiation and alkylators cause muscle loss, while novel immunotherapy is tumor-specific but their impact on cachexia is unknown. We characterize the changes in body composition in patients with relapsed/refractory myeloma treated with BCMA bispecific antibody teclistamab and determine the association between body composition with treatement response and overall survival.
Methods: 49 MM patients treated with teclistamab between 2000 and 2021 were included. All were with relapsed/refractory disease after > 4 lines of therapy and received an approved dose and schedule of teclistamab. The response assessment was according to IMWG criteria. Body weight, body mass index (BMI), tumor burden, and organ function were assessed at baseline and post-treatment. Standard-of-care abdominopelvic CT or PET/CT scans at baseline and at last known follow up were analyzed with Data Analysis Facilitation Suite (DAFS) software to quantify skeletal muscle (SKM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT). Differences between groups were tested using the χ 2 test or paired t-test. Progression free survival (PFS) and overall survival (OS) from the start of teclistamab to progression and death or last follow-up, respectively, were analyzed by the Kaplan-Meier method. Comparisons were made with the log-rank test.
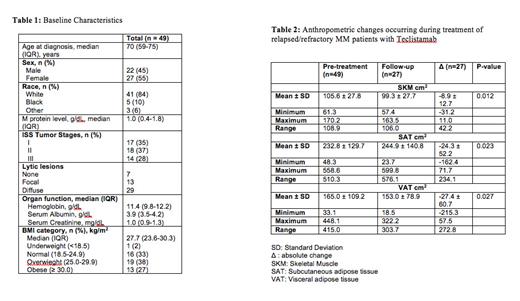
Results: Patient characteristics are detailed in Table 1. Based on the WHO definition, 2% were underweight, 16% were normal weight, 19% were overweight and 13% were obese, at baseline. The baseline mean SKM was 105.6 ± 27.8 (Male 122.0 ± 25.3, female 93.4 ± 23.5). SAT and VAT varied widely. Median SAT was 196.5 (range 48.3-558.6), and median VAT was 136.6 (33.09-448.1). Of 32 patients in the obese group, 16 (50%) patients had SKM that was below the mean SKM of the group. Baseline SAT and VAT, but not SKM were correlated with BMI. Twenty-seven of the 49 patients had paired imaging. The absolute changes in skeletal muscle and adipose tissue compartments are shown in Table 2. A majority of patients had a decline in SKM, SAT and VAT post-treatment: mean SKM decrease was 8.9 cm 2 ( p ≤ 0.01), mean SAT decreased 24.3 cm 2 ( p = 0.02) and mean VAT decreased 27.4 cm 2 ( p = 0.03), while BMI and weight were unchanged. Nineteen of 27 (70%) subjects responded to therapy. The changes in SKM, VAT, and SAT loss did not differ significantly between responders and non-responders. 38.8% of patients (19/49) had died by the end of the follow-up. Median follow-up duration was 2.8 years, overall survival was 1.7 years and PFS was 0.9 years. Baseline SKM, SAT, and VAT did not correlate with PFS or OS, but a decline in SMK significantly correlated with a shorter OS (p=0.04); 7/18 patients with decreased SKM and 2/9 patients with unchanged or increased SKM died. Response correlated strongly with overall survival (p=0.002).
Conclusions: Body composition analysis of standard CT images may provide clinically relevant information for patients with advanced myeloma. Sarcopenia was common despite high BMI, and a further decline in skeletal muscle mass correlated with shorter survival, regardless of response to tumor-specific immunotherapy. Anthropometric changes should be validated in larger patient cohorts and included in future clinical trials. Further efforts should focus on the maintenance of muscle and visceral adipose tissue in this patient population.
Disclosures
Abonour:BMS, Janssen, Takeda: Consultancy; BMS, Janssen, Pfizer: Honoraria. Suvannasankha:Bristol Meyer Squibb: Consultancy, Research Funding; Genentech: Research Funding; Janssen: Consultancy, Research Funding; Glaxo Smith Kline: Consultancy, Research Funding; Regeneron: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal